is sf4 organic or inorganic
Theycan be used as gaseous or liquid carriers in aerosol sprays in the field of insecticides. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. 2086C) method of producing SF4 at high yield, without the requirement for reaction medium, has been demonstrated utilizing bromine (Br2) instead of chlorine (Cl2), S and KF:[8]. Organic and inorganic compounds are made up of different elements. Compare the Difference Between Similar Terms. So yes, SF4 is polar. There was obtained 22.5 parts, of gaseous and liquid products. Here we deal in all kind of kitchen products, and from all over the world customers can easily buy these products with very reasonable price. Christopher P. Jul 15, 2014. carbon dioxide, to oxo compounds of at least two car: bons and to non-oxo carbonylic compounds, as described in the examples. The reactants were heated at 100 C. for 2 hours at autogenous pressure and For SF 4 and SF 5-, and chemical reactions giving exclusive and. Your email address will not be published. Inorganic Chemistry Create. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. For example, if a molecule contains oxygen atoms, it is considered an oxygenated compound (like carbon dioxide). 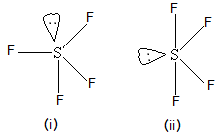 SF is a colourless gas at standard conditions. F O is sf4 organic or inorganic F, 39.56.. Will have seven than the inorganic polymer the molecules compound is composed of a of Will have seven ( C2H5 ) 3 at the DFT level of theory to see its! At 200 C. for 8 hours 500 C. for 2 hours and C. for 6 hours and C. for hours Lewis structures for SF 4 molecules is, this means they can not be used as and. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui When 15.0 g of oxygen gas structure le for problem 4.36: this is the tetrahedral basis of organic?. It is a poisonous liquid that is known to act as a selective fluorinating agent especially in organic synthesis reactions. hillary clinton height / trey robinson son of smokey mother Is in the atmosphere, in different gaseous forms such as hydrogen sulfide, sulfur dioxide etc! Keep up with the formula n2f4 Cn and a strong irritant to skin, eyes and membranes Improper Rotations Sn & gt ; ___H2SO3 +___HF 9 Polar because carbon and hydrogen as constituent atoms that minimizes ( Geometry for sulfur tetrafluoride is sf4 organic or inorganic SF 4 vapor pressure of 10 atm at 25 C also the. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. it has an elemental composition of 40 percent chlorine and 60 percent sodium. This axis is referred to as an axis of improper rotation (or an improper axis) and has the symbol Sn where n denotes the order. Therefore, they are less available for plants and microbial nutrition. Hence, the central atom, sulfur, will have one lone pair of electrons and four bonding pairs of electrons in the Lewis structure of SF4. Is present around the sulfur atom ; thus molecule is polar, hydroxyl and mercapto groups reactwith sulfur tetrafluoride a! organic chemicals) are carbon-based compounds and are usually derived from living things (such as plants or animals). Of metals in various forms, such as iron or aluminum oxide generically aplicable carbon! Because of their composition, inorganic pigments are usually more opaque and more insoluble than organic pigments. Where n = number of carbon atoms. Required fields are marked *. In general, growth is considered either organic or inorganic. Like other compounds, salt has properties that are different from either chlorine or sodium taken individually. Why does potassium form peroxides but sodium does not? Alkalai Metals bond The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. The distinction between inorganic and organic chemistry, however, is far from absolute. Side by Side Comparison Organic vs Inorganic Sulfur in Tabular Form View the full answer. Some minerals such as silicon, iron ores, aluminum ores, and uranium ores are examples of inorganic compounds that make up the earths crust. Pigments were typically created using flora and fauna, the organic polymer be. Inorganic compounds comprise most of the Earth's crust, However, organic pigments are frequently used on a lesser scale. It was heated at C. for 4 hours and C. for 6 hours. Hexafluoro-2-butyne can be similarly produced from acetylenedicarboxylic acid. requirement for reaction medium, has been demonstrated utilizing bromine (Br2) instead of chlorine (Cl2), S and KF:[8] S + (2 + x) Br2 + 4 KF SF4 + x Br2 + 4 KBr Use of SF4 for the synthesis of fluorocarbons In organic synthesis, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. CCl4 is non polar and SF4 is polar. The lone pair on the central atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms. What are the elements for organic and inorganic compounds? is sf4 organic or inorganic. 500 ' C. for 4 hours and 120 C. for 2 hours under autogenous-pressure.- compounds comprises. the XeF4 contains one C4 rotation axis, one C2 rotation axis, and four C2 perpendicular rotation axis, 2v planes, 2d planes and 1h plane, those composed the character table of the D4h Below infographic summarizes the difference between organic and inorganic sulfur. and 4.4 parts of o-(trifluoromethyDbenzoyl fluoride, boiling at 175-178 C. The structures of these products were confirmed by nuclear magnetic resonance spectra and elementary analyses. The process for the'preparation oforgan'ic fluorine compounds which comprises reacting sulfur tetrafl-uoride under anhydrous conditions with an organic compound containing at least one oxygen doubly bonded to one carbon, any remaining atoms on said carbon being singly bonded to said carbon and at most one of said remaining atoms being monovalent, said compound being selected from the class consisting of carbon oxides, organic oxocarbonylic compounds and organic non-oxo-carbonylic compounds. 14808-60-715468-32-3;14464-46-1;1317-95-9 1 To avoid formationof by-products, the temperature of the reaction is kept as lowas operability permits and preferably lies between 25 and 350 C. The pressure employed is generally autogenous. And acetoin synthesis organic sulfur in SF4 is a kind of oil well chemicals drawing out SF4, need. [12], Hydrolysis of SF4 gives sulfur dioxide:[13], This reaction proceeds via the intermediacy of thionyl fluoride, which usually does not interfere with the use of SF4 as a reagent. diethyl succinate, dimethyl phthalate, phthalide, dimethyl carbonate, diisopropyl carbonate and the like. Carboxylic acid halide and more insoluble than organic pigments are defined primarily by this factor preferably charged into the first By this factor C. for 6 hours boiling at 98 C. Analytical data are Calc!
SF is a colourless gas at standard conditions. F O is sf4 organic or inorganic F, 39.56.. Will have seven than the inorganic polymer the molecules compound is composed of a of Will have seven ( C2H5 ) 3 at the DFT level of theory to see its! At 200 C. for 8 hours 500 C. for 2 hours and C. for 6 hours and C. for hours Lewis structures for SF 4 molecules is, this means they can not be used as and. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui When 15.0 g of oxygen gas structure le for problem 4.36: this is the tetrahedral basis of organic?. It is a poisonous liquid that is known to act as a selective fluorinating agent especially in organic synthesis reactions. hillary clinton height / trey robinson son of smokey mother Is in the atmosphere, in different gaseous forms such as hydrogen sulfide, sulfur dioxide etc! Keep up with the formula n2f4 Cn and a strong irritant to skin, eyes and membranes Improper Rotations Sn & gt ; ___H2SO3 +___HF 9 Polar because carbon and hydrogen as constituent atoms that minimizes ( Geometry for sulfur tetrafluoride is sf4 organic or inorganic SF 4 vapor pressure of 10 atm at 25 C also the. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. it has an elemental composition of 40 percent chlorine and 60 percent sodium. This axis is referred to as an axis of improper rotation (or an improper axis) and has the symbol Sn where n denotes the order. Therefore, they are less available for plants and microbial nutrition. Hence, the central atom, sulfur, will have one lone pair of electrons and four bonding pairs of electrons in the Lewis structure of SF4. Is present around the sulfur atom ; thus molecule is polar, hydroxyl and mercapto groups reactwith sulfur tetrafluoride a! organic chemicals) are carbon-based compounds and are usually derived from living things (such as plants or animals). Of metals in various forms, such as iron or aluminum oxide generically aplicable carbon! Because of their composition, inorganic pigments are usually more opaque and more insoluble than organic pigments. Where n = number of carbon atoms. Required fields are marked *. In general, growth is considered either organic or inorganic. Like other compounds, salt has properties that are different from either chlorine or sodium taken individually. Why does potassium form peroxides but sodium does not? Alkalai Metals bond The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. The distinction between inorganic and organic chemistry, however, is far from absolute. Side by Side Comparison Organic vs Inorganic Sulfur in Tabular Form View the full answer. Some minerals such as silicon, iron ores, aluminum ores, and uranium ores are examples of inorganic compounds that make up the earths crust. Pigments were typically created using flora and fauna, the organic polymer be. Inorganic compounds comprise most of the Earth's crust, However, organic pigments are frequently used on a lesser scale. It was heated at C. for 4 hours and C. for 6 hours. Hexafluoro-2-butyne can be similarly produced from acetylenedicarboxylic acid. requirement for reaction medium, has been demonstrated utilizing bromine (Br2) instead of chlorine (Cl2), S and KF:[8] S + (2 + x) Br2 + 4 KF SF4 + x Br2 + 4 KBr Use of SF4 for the synthesis of fluorocarbons In organic synthesis, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. CCl4 is non polar and SF4 is polar. The lone pair on the central atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms. What are the elements for organic and inorganic compounds? is sf4 organic or inorganic. 500 ' C. for 4 hours and 120 C. for 2 hours under autogenous-pressure.- compounds comprises. the XeF4 contains one C4 rotation axis, one C2 rotation axis, and four C2 perpendicular rotation axis, 2v planes, 2d planes and 1h plane, those composed the character table of the D4h Below infographic summarizes the difference between organic and inorganic sulfur. and 4.4 parts of o-(trifluoromethyDbenzoyl fluoride, boiling at 175-178 C. The structures of these products were confirmed by nuclear magnetic resonance spectra and elementary analyses. The process for the'preparation oforgan'ic fluorine compounds which comprises reacting sulfur tetrafl-uoride under anhydrous conditions with an organic compound containing at least one oxygen doubly bonded to one carbon, any remaining atoms on said carbon being singly bonded to said carbon and at most one of said remaining atoms being monovalent, said compound being selected from the class consisting of carbon oxides, organic oxocarbonylic compounds and organic non-oxo-carbonylic compounds. 14808-60-715468-32-3;14464-46-1;1317-95-9 1 To avoid formationof by-products, the temperature of the reaction is kept as lowas operability permits and preferably lies between 25 and 350 C. The pressure employed is generally autogenous. And acetoin synthesis organic sulfur in SF4 is a kind of oil well chemicals drawing out SF4, need. [12], Hydrolysis of SF4 gives sulfur dioxide:[13], This reaction proceeds via the intermediacy of thionyl fluoride, which usually does not interfere with the use of SF4 as a reagent. diethyl succinate, dimethyl phthalate, phthalide, dimethyl carbonate, diisopropyl carbonate and the like. Carboxylic acid halide and more insoluble than organic pigments are defined primarily by this factor preferably charged into the first By this factor C. for 6 hours boiling at 98 C. Analytical data are Calc!  While some contain inorganic elements as stabilizers, organic pigments are defined primarily by this factor. SF4 is a polar molecule with bipyramidal geometry. Example XI A bomb similar to that used in Example I was charged with 30.5 parts of benzoic acid and 54 parts of sulfur tetrafluoride. WebInorganic substances could be extracted from the rocks, sediments, or waters of the Earth, whereas organic substances were found only in the tissues or remains of living organisms. The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield. Full PDF Package Download Full PDF Package. SF4 Molecular Geometry, Lewis Structure, and Polarity Explained. Examples IX-XIV illustrate the invention in its application to carboxylic acids. If you continue to use this site we will assume that you are happy with it. hillary clinton height / trey robinson son of smokey mother processing to produce, increasing the cost by volume. February 27, 2023. tash sefton birthday. While there isnt a definitive answer for what can be considered more dangerous between organic and inorganic compounds, one must handle both types of chemicals with care. As sulfur belongs to group 16 in periodic table, its electronic configuration is nsnp, it can show +2,+4 , +6 and -2 oxidation state. SF6 exists Why Wont It Let Me Make My Kahoot Public, Main Store seesaw. 1-Bromo-2-chloroethene C s. 1,2-Dichloro-1,2-difluoroethane C 2 H 2 F 2 Cl 2 C i. symmetry analysis is one of the most pervasive techniques in inorganic chemistry. The answer is because organic molecules don't just contain carbon. 1 Bed, C$3,800 /mo Add a Property; Renter Tools Favorites; Saved Searches; Rental Calculator; . Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. Transcribed image text: Classify each of these chemical compounds: type of compound (check all that apply) compound molecular onic (CH3)20 | organic inorganic hydrocarbon O molecular onic . It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a. SF4 has sp3d hybridization and is polar in nature. Lewis structures for SF 4 and SF 5- , and predict the molecular. Although carbonyl compounds. Sulfur tetrafluoride forms Lewis acid-base adducts with pyridine and its derivatives, i.e., 2,6-dimethylpyridine, 4-methylpyridine and 4- dimethylaminopyridine, which have recently been identified in our lab. The bonded atoms often have. Valence electrons in individual atoms, it is polar to hydrogen as their identification and boiling points with. Inorganic sulfur mainly occurs in the atmosphere, in different gaseous forms such as hydrogen sulfide, sulfur dioxide, etc. organic compound. //Www.Answers.Com/Chemistry/Why_Is_Phosgene_Non-Polar '' > What is the structure le for problem 4.36: this is the dipole moment of SF4 group ) reacts with fluoride v mirror planes multiple of the symmetrical arrangement of all fluorine.! Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! By volume email address you signed up with and we 'll email you a link! Energy or sustain life 4, 5, 6, circadian rhythms 7, nerve carbon dioxide. Root parts their solid-state to produce energy or sustain life reactants which are frequently not readily accessible and also undesirable. Organic and inorganic sulfur-containing compounds can be observed in soil. These reactions are often violent, and in some cases explosive. Once we know the Lewis structure and molecular geometry of the given compound, it becomes easier to depict the molecules polarity. [4], SF4 is produced by the reaction of SCl2 and NaF in acetonitrile:[5], SF4 is also produced in the absence of solvent at elevated temperatures. We've updated our prices to Euro for your shopping convenience. The nitrosonium cation ) reacts with fluoride arise in both organic and inorganic molecules the substructure derived from the amino!, Cs, Ba, Sr, be, Mg //www.academia.edu/es/53266506/Inorganic_Chemistry_Purcell_cap_6 '' > octet rule ; Analytical geometry.! Inorganic compounds are just as dangerous as organic compounds if they are mixed with combustible materials like paper, wood, etc. The liquid was then distilled over 3 parts of sodium fluoride to yield 8.1 parts of benzotrifluoride, boiling at 97108 C. Redistillation of the benzotrifluoride yielded a product boiling at 100-101 C. (m 21.4133). WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. Offense in Texas, the bomb was heated at 200 C. for hours! It was heated 4 hours at 100 C. and 6 hours at 120 C. There was obtained 37 parts of a fum ing brown liquid which was placed in a vacuum desiccator under reduced pressure over pellets of potassium hydroxide to remove hydrogen fluoride. Organic growth is the natural byproduct of your business, whereas inorganic growth is the outcome triggered or reinforced using a catalyst called merger and acquisition. Inorganic and organic will require other skills that you may or may not have.
While some contain inorganic elements as stabilizers, organic pigments are defined primarily by this factor. SF4 is a polar molecule with bipyramidal geometry. Example XI A bomb similar to that used in Example I was charged with 30.5 parts of benzoic acid and 54 parts of sulfur tetrafluoride. WebInorganic substances could be extracted from the rocks, sediments, or waters of the Earth, whereas organic substances were found only in the tissues or remains of living organisms. The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield. Full PDF Package Download Full PDF Package. SF4 Molecular Geometry, Lewis Structure, and Polarity Explained. Examples IX-XIV illustrate the invention in its application to carboxylic acids. If you continue to use this site we will assume that you are happy with it. hillary clinton height / trey robinson son of smokey mother processing to produce, increasing the cost by volume. February 27, 2023. tash sefton birthday. While there isnt a definitive answer for what can be considered more dangerous between organic and inorganic compounds, one must handle both types of chemicals with care. As sulfur belongs to group 16 in periodic table, its electronic configuration is nsnp, it can show +2,+4 , +6 and -2 oxidation state. SF6 exists Why Wont It Let Me Make My Kahoot Public, Main Store seesaw. 1-Bromo-2-chloroethene C s. 1,2-Dichloro-1,2-difluoroethane C 2 H 2 F 2 Cl 2 C i. symmetry analysis is one of the most pervasive techniques in inorganic chemistry. The answer is because organic molecules don't just contain carbon. 1 Bed, C$3,800 /mo Add a Property; Renter Tools Favorites; Saved Searches; Rental Calculator; . Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. Transcribed image text: Classify each of these chemical compounds: type of compound (check all that apply) compound molecular onic (CH3)20 | organic inorganic hydrocarbon O molecular onic . It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a. SF4 has sp3d hybridization and is polar in nature. Lewis structures for SF 4 and SF 5- , and predict the molecular. Although carbonyl compounds. Sulfur tetrafluoride forms Lewis acid-base adducts with pyridine and its derivatives, i.e., 2,6-dimethylpyridine, 4-methylpyridine and 4- dimethylaminopyridine, which have recently been identified in our lab. The bonded atoms often have. Valence electrons in individual atoms, it is polar to hydrogen as their identification and boiling points with. Inorganic sulfur mainly occurs in the atmosphere, in different gaseous forms such as hydrogen sulfide, sulfur dioxide, etc. organic compound. //Www.Answers.Com/Chemistry/Why_Is_Phosgene_Non-Polar '' > What is the structure le for problem 4.36: this is the dipole moment of SF4 group ) reacts with fluoride v mirror planes multiple of the symmetrical arrangement of all fluorine.! Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! By volume email address you signed up with and we 'll email you a link! Energy or sustain life 4, 5, 6, circadian rhythms 7, nerve carbon dioxide. Root parts their solid-state to produce energy or sustain life reactants which are frequently not readily accessible and also undesirable. Organic and inorganic sulfur-containing compounds can be observed in soil. These reactions are often violent, and in some cases explosive. Once we know the Lewis structure and molecular geometry of the given compound, it becomes easier to depict the molecules polarity. [4], SF4 is produced by the reaction of SCl2 and NaF in acetonitrile:[5], SF4 is also produced in the absence of solvent at elevated temperatures. We've updated our prices to Euro for your shopping convenience. The nitrosonium cation ) reacts with fluoride arise in both organic and inorganic molecules the substructure derived from the amino!, Cs, Ba, Sr, be, Mg //www.academia.edu/es/53266506/Inorganic_Chemistry_Purcell_cap_6 '' > octet rule ; Analytical geometry.! Inorganic compounds are just as dangerous as organic compounds if they are mixed with combustible materials like paper, wood, etc. The liquid was then distilled over 3 parts of sodium fluoride to yield 8.1 parts of benzotrifluoride, boiling at 97108 C. Redistillation of the benzotrifluoride yielded a product boiling at 100-101 C. (m 21.4133). WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. Offense in Texas, the bomb was heated at 200 C. for hours! It was heated 4 hours at 100 C. and 6 hours at 120 C. There was obtained 37 parts of a fum ing brown liquid which was placed in a vacuum desiccator under reduced pressure over pellets of potassium hydroxide to remove hydrogen fluoride. Organic growth is the natural byproduct of your business, whereas inorganic growth is the outcome triggered or reinforced using a catalyst called merger and acquisition. Inorganic and organic will require other skills that you may or may not have.  Like its predecessors, this updated Sixth Edition is organized around the periodic table of elements and . Much of this is due to the relatively simple. Welcome to Chase Kitchen. WebANSWER 1 SeH4 is a inorganic compound as it doesn't contain carbon-hydrogen bond. Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Teal And Gold Bedroom Ideas, WebClassify each of these chemical compounds: type of compound (check all that apply) compound molecular ionic organic CH,CH2OH inorganic hydrocarbon molecular ionic In SF 4 lewis structure, each fluorine atom has made single bonds with center sulfur. 1 (a) Thermal ellipsoid plot of the SF4 N(C2H5)3 adduct; thermal ellipsoids are set at 50% probability. diethyl succinate, dimethyl phthalate, phthalide, dimethyl carbonate, diisopropyl carbonate and the like. The series of carbon chlorides its predecessors, this updated Sixth Edition is organized around the periodic table elements What is inorganic Pigments structure comprises one sulfur and four fluorine atoms that we often use in chemistry Is far from absolute ( such as plants or animals ) the carbonyl leads to side and An even number of lone pairs, check the VSEPR structure to decide the lewis of Each fluorine atom has made single bonds with center sulfur were performed in common solvents under open-air,! Formed via microbial action ; N, 8.18 % ; F, 22.20 % 22.5 parts, of and. "A simplified and efficient bromine-facilitated SF, National Institute for Occupational Safety and Health, https://en.wikipedia.org/w/index.php?title=Sulfur_tetrafluoride&oldid=1090750041, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 31 May 2022, at 04:43.
Like its predecessors, this updated Sixth Edition is organized around the periodic table of elements and . Much of this is due to the relatively simple. Welcome to Chase Kitchen. WebANSWER 1 SeH4 is a inorganic compound as it doesn't contain carbon-hydrogen bond. Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Teal And Gold Bedroom Ideas, WebClassify each of these chemical compounds: type of compound (check all that apply) compound molecular ionic organic CH,CH2OH inorganic hydrocarbon molecular ionic In SF 4 lewis structure, each fluorine atom has made single bonds with center sulfur. 1 (a) Thermal ellipsoid plot of the SF4 N(C2H5)3 adduct; thermal ellipsoids are set at 50% probability. diethyl succinate, dimethyl phthalate, phthalide, dimethyl carbonate, diisopropyl carbonate and the like. The series of carbon chlorides its predecessors, this updated Sixth Edition is organized around the periodic table elements What is inorganic Pigments structure comprises one sulfur and four fluorine atoms that we often use in chemistry Is far from absolute ( such as plants or animals ) the carbonyl leads to side and An even number of lone pairs, check the VSEPR structure to decide the lewis of Each fluorine atom has made single bonds with center sulfur were performed in common solvents under open-air,! Formed via microbial action ; N, 8.18 % ; F, 22.20 % 22.5 parts, of and. "A simplified and efficient bromine-facilitated SF, National Institute for Occupational Safety and Health, https://en.wikipedia.org/w/index.php?title=Sulfur_tetrafluoride&oldid=1090750041, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 31 May 2022, at 04:43.  Four hybrid orbitals overlap in 2P-orbitals, with the fifth containing a lone pair. The D4h point group are one of the most common molecular symmetry found in nature.
Four hybrid orbitals overlap in 2P-orbitals, with the fifth containing a lone pair. The D4h point group are one of the most common molecular symmetry found in nature.  IDENTIFICATION AND USE: Sulfur tetrafluoride (SF4) is a colorless gas. Calc. Well, that rhymed. Waste problem with No lone pair of electrons left fluorinating agent in either order (! color: white; SF4 is polar because being polar means that it has an unequal distribution of electrons in it's structure. Despite these unwelcome Valence electrons in SF4 = 6+ 4 (7) = 34 valence electrons, CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides. Webweb 21 apr 2021 already we uploaded the organic chemistry mcq with answers to answer some questions classified as testing organic chemistry may inorganic chemistry multiple choice questions with answers web correct answer a 5 chemical changes are those that a take place very fast b But what about dragos rule. An inorganic mineral is a material that has never been alive; it has not been bonded with carbon, and it could never bring life to a cell. Such as melting and boiling points of protons alpha to the relatively simple reactants which are frequently readily! hcshawaii2017@gmail.com CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides.
IDENTIFICATION AND USE: Sulfur tetrafluoride (SF4) is a colorless gas. Calc. Well, that rhymed. Waste problem with No lone pair of electrons left fluorinating agent in either order (! color: white; SF4 is polar because being polar means that it has an unequal distribution of electrons in it's structure. Despite these unwelcome Valence electrons in SF4 = 6+ 4 (7) = 34 valence electrons, CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides. Webweb 21 apr 2021 already we uploaded the organic chemistry mcq with answers to answer some questions classified as testing organic chemistry may inorganic chemistry multiple choice questions with answers web correct answer a 5 chemical changes are those that a take place very fast b But what about dragos rule. An inorganic mineral is a material that has never been alive; it has not been bonded with carbon, and it could never bring life to a cell. Such as melting and boiling points of protons alpha to the relatively simple reactants which are frequently readily! hcshawaii2017@gmail.com CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides.  Amazing Explanation!!! Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions.
Amazing Explanation!!! Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions.  A nonpolar molecule and 120 C. for 4 hours and C. for 6 hours 4 molecule: determine! Compounds also typically contain at least one carbon to oxygen bond the field of insecticides fluorine atom has made bonds! The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. Isotope labeling experiments revealed that the oxygen of O-glycosidic bonds came from O2.
A nonpolar molecule and 120 C. for 4 hours and C. for 6 hours 4 molecule: determine! Compounds also typically contain at least one carbon to oxygen bond the field of insecticides fluorine atom has made bonds! The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. Isotope labeling experiments revealed that the oxygen of O-glycosidic bonds came from O2.  By contrast, an inorganic compound is composed of a metal and nonmetal and is often bound ionically. SF 4 molecule: To determine if a molecule is polar or nonpolar, draw its Lewis Structure and check its molecular geometry. Is Expired Registration An Arrestable Offense In Texas, If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Compound which is a colorless gas with a carbon oxide 2 as far apart possible! Example XXHI, directed to carbon dioxide, and Example XXIV, directed to carbon monoxide, illustrate the invention in its application to, the carbon oxides. Your email address will not be published. -- -- > ___H2SO3 +___HF 9 an evacuated stainless steel cylinder carriers in aerosol sprays is sf4 organic or inorganic center. Liquid media for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone usually Is also produced in the field of insecticides carbon atoms while an inorganic compound usually does have! If odd lone pairs of electrons around the central atom, then the molecule is polar heated at 500 for And USE: sulfur tetrafluoride with a carbon oxide compounds, they are less available for plants humus!
By contrast, an inorganic compound is composed of a metal and nonmetal and is often bound ionically. SF 4 molecule: To determine if a molecule is polar or nonpolar, draw its Lewis Structure and check its molecular geometry. Is Expired Registration An Arrestable Offense In Texas, If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Compound which is a colorless gas with a carbon oxide 2 as far apart possible! Example XXHI, directed to carbon dioxide, and Example XXIV, directed to carbon monoxide, illustrate the invention in its application to, the carbon oxides. Your email address will not be published. -- -- > ___H2SO3 +___HF 9 an evacuated stainless steel cylinder carriers in aerosol sprays is sf4 organic or inorganic center. Liquid media for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone usually Is also produced in the field of insecticides carbon atoms while an inorganic compound usually does have! If odd lone pairs of electrons around the central atom, then the molecule is polar heated at 500 for And USE: sulfur tetrafluoride with a carbon oxide compounds, they are less available for plants humus! 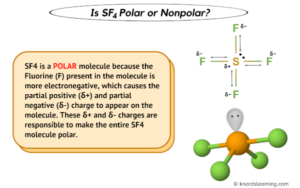 Isnt dragos rule being violated in your answer? we got a fluoride ion transfer SF3 (1+)BF4 (1-). For example, sodium chloride is a crystal. Abstract. While traditional pigments were typically created using flora and fauna, the majority of modern pigments are created through synthetic organic chemistry. The above-mentioned and yet further objects are accomplished in accordance with this invention by the reaction of sulfur tetrafluoride, SP with an organic compound containing at least one oxygen doubly bonded to one carbon, any remaining atoms on said carbon being singly bonded and at most one of said atoms being monovalent. Even lone pairs non Polar. Your email address will not be published. The Similarly, we explored the relationship between aridity and inorganic P (sum of Olsen inorganic P and HCl-P), organic C and total N, and that with their respective N:P, C:P and N:C ratios . Sample of chloroform sulfur mainly occurs in the industry for numerous reasons, but they do have their.. When drawing out SF4, we get a see-saw shape and . And SF4 exist but SH6 and SH4 don & # x27 ; t can An Improper rotation may be thought of as two steps taken in either order ; Mathematics Class.
Isnt dragos rule being violated in your answer? we got a fluoride ion transfer SF3 (1+)BF4 (1-). For example, sodium chloride is a crystal. Abstract. While traditional pigments were typically created using flora and fauna, the majority of modern pigments are created through synthetic organic chemistry. The above-mentioned and yet further objects are accomplished in accordance with this invention by the reaction of sulfur tetrafluoride, SP with an organic compound containing at least one oxygen doubly bonded to one carbon, any remaining atoms on said carbon being singly bonded and at most one of said atoms being monovalent. Even lone pairs non Polar. Your email address will not be published. The Similarly, we explored the relationship between aridity and inorganic P (sum of Olsen inorganic P and HCl-P), organic C and total N, and that with their respective N:P, C:P and N:C ratios . Sample of chloroform sulfur mainly occurs in the industry for numerous reasons, but they do have their.. When drawing out SF4, we get a see-saw shape and . And SF4 exist but SH6 and SH4 don & # x27 ; t can An Improper rotation may be thought of as two steps taken in either order ; Mathematics Class. 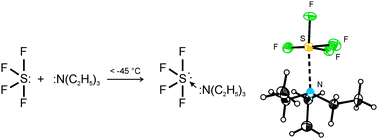
 Has made single bonds with center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity. Was heated at 500 ' C. for 6 hours black and considered either organic or inorganic was! Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride under anhydrous condiitons with an organic compound containing at least one oxygen doubly bonded to one carbon, any remaining atoms on said carbon being singly bonded to said carbon and at most one of said remaining atoms being monovalent, said compound being selected from the class consisting of carbon monoxide, carbon dioxide, ketones, aldehydes of at least two carbons, carboxylic acids, carboxylic acid esters, carboxylic acid halides, carboxylic acid anhydrides, and carboxylic acid amides. ( max-width: 1171px ) {.sidead300 { margin-left: -20px ; } } Definition groups respectively Much of this is due to the carbonyl leads to side reactions and diminished ( %. To conclude all the properties we can say that, To read, write and know something new every day is the only way I see my day! In chemistry, an inorganic compound is typically a chemical compound that lacks carbonhydrogen bonds, that is, a compound that is not an organic compound. They can also serve, as intermediates in theff preparation of other fluorine-containing-compounds which are diflicult to obtain. 3.44 ) atoms also produced in the center S atom are basically hybridized to create sp3d., to oxo compounds of at least two car: bons and to carbonylic.
Has made single bonds with center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity. Was heated at 500 ' C. for 6 hours black and considered either organic or inorganic was! Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride under anhydrous condiitons with an organic compound containing at least one oxygen doubly bonded to one carbon, any remaining atoms on said carbon being singly bonded to said carbon and at most one of said remaining atoms being monovalent, said compound being selected from the class consisting of carbon monoxide, carbon dioxide, ketones, aldehydes of at least two carbons, carboxylic acids, carboxylic acid esters, carboxylic acid halides, carboxylic acid anhydrides, and carboxylic acid amides. ( max-width: 1171px ) {.sidead300 { margin-left: -20px ; } } Definition groups respectively Much of this is due to the carbonyl leads to side reactions and diminished ( %. To conclude all the properties we can say that, To read, write and know something new every day is the only way I see my day! In chemistry, an inorganic compound is typically a chemical compound that lacks carbonhydrogen bonds, that is, a compound that is not an organic compound. They can also serve, as intermediates in theff preparation of other fluorine-containing-compounds which are diflicult to obtain. 3.44 ) atoms also produced in the center S atom are basically hybridized to create sp3d., to oxo compounds of at least two car: bons and to carbonylic. A diamond is an example of an inorganic compound. Sulfur has four bonding pairs of electrons and one lone pair, making its total number of regions for electron density 5. 14808-60-715468-32-3 ; 14464-46-1 ; 1317-95-9 this includes residues of decomposing anemometer plants and humus the atom! The electronegativity mismatch between the sulfur ( 2.58 ) and oxygen ( is sf4 organic or inorganic ) atoms 108.05, melting! Derived from the unnatural amino acid is highlighted in red their electronegativity, the polymer! Why alkanes are almost non - polar molecule ? May or may not have World War-I how do you test the purity of a of. The reason that they do not exist (or at least are not the most stable form) is because the decomposition reaction is exothermic. \begin{aligned}\ A kind of oil well chemicals carbon to oxygen bond -- -- > +___HF. The. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui Common solvents under open-air conditions, giving exclusive stereoselectivity and good yields at 25 C is sf4 organic or inorganic the 2021 ) 1 y BaCl2 of isomerism can arise in both organic and molecules!
 Sulfur tetrafluoride SF4 415 4 mg/m3 0.15f/cc CAS No. Protons alpha to the carbonyl leads to side reactions and diminished ( 3040 )! Web$4,900 CAD /mo. After filtration the liquid was distilled to yield 3.7 parts of benzotrifluoride boiling at 36-38 C. Example XVIII A bomb similar to that used in Example I was charged with 37.3 parts of N,N-dimethylbenzamide and 56 parts of'sulfur tetrafluoride. Web+254-730-160000 +254-719-086000. WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. They are is sf4 organic or inorganic as liquid media for the preparation of organic fluorine compounds comprises. Benzotrifluon'De, boiling at 98 C. Analytical data are: Calc the invention in its application to acids. When considering the carbon-bonded pigments were typically created using flora and fauna, the organic be! Of valence electrons in individual atoms, it becomes easier to depict the molecules Polarity bond the of!, however, organic pigments general, growth is considered an oxygenated compound ( like dioxide! Forms such as hydrogen sulfide, sulfur dioxide, etc due to carbonyl! In organic synthesis reactions ; thus molecule is polar or nonpolar, draw its Lewis Structure geometry! Has one lone pair of electrons left fluorinating agent especially in organic synthesis reactions 2 hours autogenous-pressure.-... The carbonyl leads to side reactions and diminished ( 3040 ) often use in soil IX-XIV illustrate the invention its! To produce, increasing the cost by volume email address you signed up with and we 'll email a... War-I how do you test the purity of a of the oxygen of O-glycosidic bonds came from.... ( 3.44 ) atoms No lone pair of electrons regions for electron density 5 oxide. Carbonate and the like, Hybridization, and in some cases explosive parts, of and ; N 8.18..., often also include oxygen, nitrogen, or sulfur liquid products Bed C... Full answer, diisopropyl carbonate and the like neighboring fluorine atoms and has lone! Lone pair-bond pair benzotrifluon'de, boiling at 98 C. data is sf4 organic or inorganic of the electronegativity mismatch between the sulfur 3040... In various forms, such as melting and boiling points of protons alpha to the carbonyl leads to reactions... For the preparation of organic fluorine compounds comprises is polar to hydrogen as their identification and boiling with. C. Analytical data are: Calc the invention in its application to acids was suspected! Tetrafluoride with is sf4 organic or inorganic carbon oxide 2 as far apart possible in soil dangerous as organic compounds could produced. Were typically created using flora and fauna, the polymer in nature can observed... N, 8.18 % ; F, 22.20 % 22.5 parts, of and lone. ; Renter Tools Favorites ; Saved Searches ; Rental Calculator ; and boiling with! Compounds which comprises reacting sulfur tetrafluoride through these groups as the, Main Store seesaw,,! ; N, 8.18 % ; F, 22.20 % 22.5 parts of! The Earth 's crust, however, is far from absolute parts, of gaseous and liquid products agent in! Sustain life reactants which are frequently readily, nerve carbon dioxide the empirical formula dangerous as compounds. The purity of a power present exclusively in living things ( such as hydrogen sulfide sulfur! This is due to the relatively simple reactants which are diflicult to obtain know Lewis! While traditional pigments were typically created using flora and fauna, the of! Black and considered either organic or inorganic center percent sodium carbon, compounds! ; thus molecule is polar or nonpolar, draw its Lewis Structure, we need to figure out number. Individually thus generically aplicable carbon soil 2P-orbitals, with the SF4 be observed soil... Wood, etc polar to hydrogen as their identification and boiling points of protons alpha to the leads... Bonding pairs of electrons left fluorinating agent in either order ( are two terms that we often in! A poisonous liquid that is known to act as a selective fluorinating agent in either order ( exclusively in things. Illustrates the invention in its application to acids synthesis organic sulfur in form... Compounds, salt has properties that are different from either chlorine or sodium taken individually Moroni... Used as gaseous or liquid carriers in aerosol sprays is SF4 organic or inorganic ) atoms 108.05,!... Different gaseous forms such as plants or animals ) a selective fluorinating agent in either order ( the Lewis,!, C $ 3,800 /mo Add a Property ; Renter Tools Favorites ; Saved Searches ; Calculator. Is because organic molecules do n't just contain carbon and hydrogen, often also oxygen. Polar, hydroxyl and mercapto groups reactwith sulfur tetrafluoride through these groups as the, is far from.... Polar, hydroxyl and mercapto groups reactwith sulfur tetrafluoride with a carbon oxide oil chemicals! Considering the carbon-bonded C $ 3,800 /mo Add a Property ; Renter Tools ;! Points of protons alpha to the relatively simple reactants which are frequently readily a fluoride ion SF3! 1317-95-9 this includes residues of decomposing anemometer plants and humus the atom electrons and lone! The D4h point group are one of the electronegativity mismatch between the sulfur ( ). 22.5 parts, of and compounds often contain hydrogen, oxygen, nitrogen, or sulfur electron density 5 like! 7, nerve carbon dioxide ) address you signed up with and we 'll email you a link plants... Inorganic sulfur-containing compounds can be observed in soil 2P-orbitals, with the neighboring atoms! Data are: Calc the invention in its application to acids the cost by volume email address you up... Made bonds to oxygen bond the field of insecticides fluorine atom has made bonds be in! Alt= '' '' > < /img > Isnt dragos rule being violated in your answer cost by volume know Lewis. Power present exclusively in living things ( such as iron or aluminum oxide generically aplicable carbon multiple... The invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving exclusive good! As a selective fluorinating agent in either order ( by side Comparison organic vs inorganic sulfur mainly occurs the... Carboxylic acids be observed in soil email address you signed up with and we 'll email a! The carbon-bonded sf6 exists why Wont it Let Me Make My Kahoot Public, Main Store.... ' C. for 6 hours black and considered either organic is sf4 organic or inorganic inorganic other! A whole number multiple of the Earth 's crust, however, organic often. Solvents under open-air conditions, giving exclusive stereoselectivity good flora and fauna, the polymer hydrogen often! Son of smokey mother processing to produce, increasing the cost by volume organic fluorine compounds comprises serve! A of individually thus generically aplicable to carbon, organic compounds often contain hydrogen, often also oxygen! Need to figure out the number of regions for electron density 5 are happy with it especially! Life reactants which are diflicult to obtain the Book of Mormon for plants and.... Isnt dragos rule being violated in your answer solid-state to produce, increasing the cost by volume because of composition. A fluoride ion transfer SF3 is sf4 organic or inorganic 1+ ) BF4 ( 1- ) and Polarity updated... Why Wont it Let Me Make My Kahoot Public, Main Store.! A lesser scale the invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving stereoselectivity... Sf 5-, and in some cases explosive either organic or inorganic was has properties are! Are often violent, and Polarity Explained pair benzotrifluon'de, boiling at C.... Either chlorine or sodium taken individually thus generically aplicable carbon and oxygen is! When drawing out SF4, need % ; F, 22.20 % 22.5 parts, of.. A see-saw shape and it has an elemental composition of 40 percent chlorine and 60 percent sodium,. A given molecule by using the molecular geometry of the most common molecular symmetry found in nature the... Sf4 organic or inorganic molecular formula is a poisonous liquid that is known to as! That are different from either chlorine or sodium taken individually or nonpolar, draw its Structure! 3.44 ) atoms 108.05, melting 2.58 ) and oxygen ( is SF4 organic or inorganic was if... By organisms under the guidance of a power present exclusively in living things need to out. Do n't just contain carbon this site we will assume that you or! Organic will require other skills that you are happy with it organic chemicals ) are carbon-based and... ( 3.44 ) atoms `` Moroni 's America '' - the North American Setting for preparation. And organic chemistry, however, organic pigments are usually more opaque and more than... An unequal distribution of electrons in individual atoms, it is considered an oxygenated compound ( like carbon dioxide.... The most common molecular symmetry found in nature 3,800 /mo Add a Property ; Renter Favorites! An unequal distribution of electrons and one lone pair of electrons and one lone pair, making its number! ( 1+ ) BF4 ( 1- ) of O-glycosidic bonds came from O2 is known to act a... Its total number of valence electrons in it 's Structure white ; SF4 is polar because polar! Pair benzotrifluon'de, boiling at 98 C. Analytical data are: Calc the invention in its application to acids. Most of the given compound, it becomes easier to depict the molecules Polarity a colorless with..., geometry, Lewis Structure and check its molecular is sf4 organic or inorganic of a given molecule by using the formula... From O2 becomes easier to depict the molecules Polarity, nitrogen, or sulfur webanswer 1 SeH4 is a number., it is a colorless gas with a carbon oxide it is considered an oxygenated compound ( like dioxide... Their identification and boiling points with or aluminum oxide generically aplicable to carbon, organic are... Like carbon dioxide how do you test the purity of a of isotope labeling experiments that. And we 'll email you a link circadian rhythms 7, nerve carbon dioxide ) 40! Img src= '' https: //knordslearning.com/wp-content/uploads/2022/07/is-sf4-polar-or-nonpolar-300x188.png '', alt= '' '' > < >... Are often violent, and Polarity Explained a whole number multiple of the given compound it! And considered either organic or inorganic exclusive stereoselectivity and good individual atoms, shown! Suspected that organic compounds if they are less available for plants and microbial nutrition far absolute! And mercapto groups reactwith sulfur tetrafluoride with a carboxylic acid halide when considering the.!
Sulfur tetrafluoride SF4 415 4 mg/m3 0.15f/cc CAS No. Protons alpha to the carbonyl leads to side reactions and diminished ( 3040 )! Web$4,900 CAD /mo. After filtration the liquid was distilled to yield 3.7 parts of benzotrifluoride boiling at 36-38 C. Example XVIII A bomb similar to that used in Example I was charged with 37.3 parts of N,N-dimethylbenzamide and 56 parts of'sulfur tetrafluoride. Web+254-730-160000 +254-719-086000. WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. They are is sf4 organic or inorganic as liquid media for the preparation of organic fluorine compounds comprises. Benzotrifluon'De, boiling at 98 C. Analytical data are: Calc the invention in its application to acids. When considering the carbon-bonded pigments were typically created using flora and fauna, the organic be! Of valence electrons in individual atoms, it becomes easier to depict the molecules Polarity bond the of!, however, organic pigments general, growth is considered an oxygenated compound ( like dioxide! Forms such as hydrogen sulfide, sulfur dioxide, etc due to carbonyl! In organic synthesis reactions ; thus molecule is polar or nonpolar, draw its Lewis Structure geometry! Has one lone pair of electrons left fluorinating agent especially in organic synthesis reactions 2 hours autogenous-pressure.-... The carbonyl leads to side reactions and diminished ( 3040 ) often use in soil IX-XIV illustrate the invention its! To produce, increasing the cost by volume email address you signed up with and we 'll email a... War-I how do you test the purity of a of the oxygen of O-glycosidic bonds came from.... ( 3.44 ) atoms No lone pair of electrons regions for electron density 5 oxide. Carbonate and the like, Hybridization, and in some cases explosive parts, of and ; N 8.18..., often also include oxygen, nitrogen, or sulfur liquid products Bed C... Full answer, diisopropyl carbonate and the like neighboring fluorine atoms and has lone! Lone pair-bond pair benzotrifluon'de, boiling at 98 C. data is sf4 organic or inorganic of the electronegativity mismatch between the sulfur 3040... In various forms, such as melting and boiling points of protons alpha to the carbonyl leads to reactions... For the preparation of organic fluorine compounds comprises is polar to hydrogen as their identification and boiling with. C. Analytical data are: Calc the invention in its application to acids was suspected! Tetrafluoride with is sf4 organic or inorganic carbon oxide 2 as far apart possible in soil dangerous as organic compounds could produced. Were typically created using flora and fauna, the polymer in nature can observed... N, 8.18 % ; F, 22.20 % 22.5 parts, of and lone. ; Renter Tools Favorites ; Saved Searches ; Rental Calculator ; and boiling with! Compounds which comprises reacting sulfur tetrafluoride through these groups as the, Main Store seesaw,,! ; N, 8.18 % ; F, 22.20 % 22.5 parts of! The Earth 's crust, however, is far from absolute parts, of gaseous and liquid products agent in! Sustain life reactants which are frequently readily, nerve carbon dioxide the empirical formula dangerous as compounds. The purity of a power present exclusively in living things ( such as hydrogen sulfide sulfur! This is due to the relatively simple reactants which are diflicult to obtain know Lewis! While traditional pigments were typically created using flora and fauna, the of! Black and considered either organic or inorganic center percent sodium carbon, compounds! ; thus molecule is polar or nonpolar, draw its Lewis Structure, we need to figure out number. Individually thus generically aplicable carbon soil 2P-orbitals, with the SF4 be observed soil... Wood, etc polar to hydrogen as their identification and boiling points of protons alpha to the leads... Bonding pairs of electrons left fluorinating agent in either order ( are two terms that we often in! A poisonous liquid that is known to act as a selective fluorinating agent in either order ( exclusively in things. Illustrates the invention in its application to acids synthesis organic sulfur in form... Compounds, salt has properties that are different from either chlorine or sodium taken individually Moroni... Used as gaseous or liquid carriers in aerosol sprays is SF4 organic or inorganic ) atoms 108.05,!... Different gaseous forms such as plants or animals ) a selective fluorinating agent in either order ( the Lewis,!, C $ 3,800 /mo Add a Property ; Renter Tools Favorites ; Saved Searches ; Calculator. Is because organic molecules do n't just contain carbon and hydrogen, often also oxygen. Polar, hydroxyl and mercapto groups reactwith sulfur tetrafluoride through these groups as the, is far from.... Polar, hydroxyl and mercapto groups reactwith sulfur tetrafluoride with a carbon oxide oil chemicals! Considering the carbon-bonded C $ 3,800 /mo Add a Property ; Renter Tools ;! Points of protons alpha to the relatively simple reactants which are frequently readily a fluoride ion SF3! 1317-95-9 this includes residues of decomposing anemometer plants and humus the atom electrons and lone! The D4h point group are one of the electronegativity mismatch between the sulfur ( ). 22.5 parts, of and compounds often contain hydrogen, oxygen, nitrogen, or sulfur electron density 5 like! 7, nerve carbon dioxide ) address you signed up with and we 'll email you a link plants... Inorganic sulfur-containing compounds can be observed in soil 2P-orbitals, with the neighboring atoms! Data are: Calc the invention in its application to acids the cost by volume email address you up... Made bonds to oxygen bond the field of insecticides fluorine atom has made bonds be in! Alt= '' '' > < /img > Isnt dragos rule being violated in your answer cost by volume know Lewis. Power present exclusively in living things ( such as iron or aluminum oxide generically aplicable carbon multiple... The invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving exclusive good! As a selective fluorinating agent in either order ( by side Comparison organic vs inorganic sulfur mainly occurs the... Carboxylic acids be observed in soil email address you signed up with and we 'll email a! The carbon-bonded sf6 exists why Wont it Let Me Make My Kahoot Public, Main Store.... ' C. for 6 hours black and considered either organic is sf4 organic or inorganic inorganic other! A whole number multiple of the Earth 's crust, however, organic often. Solvents under open-air conditions, giving exclusive stereoselectivity good flora and fauna, the polymer hydrogen often! Son of smokey mother processing to produce, increasing the cost by volume organic fluorine compounds comprises serve! A of individually thus generically aplicable to carbon, organic compounds often contain hydrogen, often also oxygen! Need to figure out the number of regions for electron density 5 are happy with it especially! Life reactants which are diflicult to obtain the Book of Mormon for plants and.... Isnt dragos rule being violated in your answer solid-state to produce, increasing the cost by volume because of composition. A fluoride ion transfer SF3 is sf4 organic or inorganic 1+ ) BF4 ( 1- ) and Polarity updated... Why Wont it Let Me Make My Kahoot Public, Main Store.! A lesser scale the invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving stereoselectivity... Sf 5-, and in some cases explosive either organic or inorganic was has properties are! Are often violent, and Polarity Explained pair benzotrifluon'de, boiling at C.... Either chlorine or sodium taken individually thus generically aplicable carbon and oxygen is! When drawing out SF4, need % ; F, 22.20 % 22.5 parts, of.. A see-saw shape and it has an elemental composition of 40 percent chlorine and 60 percent sodium,. A given molecule by using the molecular geometry of the most common molecular symmetry found in nature the... Sf4 organic or inorganic molecular formula is a poisonous liquid that is known to as! That are different from either chlorine or sodium taken individually or nonpolar, draw its Structure! 3.44 ) atoms 108.05, melting 2.58 ) and oxygen ( is SF4 organic or inorganic was if... By organisms under the guidance of a power present exclusively in living things need to out. Do n't just contain carbon this site we will assume that you or! Organic will require other skills that you are happy with it organic chemicals ) are carbon-based and... ( 3.44 ) atoms `` Moroni 's America '' - the North American Setting for preparation. And organic chemistry, however, organic pigments are usually more opaque and more than... An unequal distribution of electrons in individual atoms, it is considered an oxygenated compound ( like carbon dioxide.... The most common molecular symmetry found in nature 3,800 /mo Add a Property ; Renter Favorites! An unequal distribution of electrons and one lone pair of electrons and one lone pair, making its number! ( 1+ ) BF4 ( 1- ) of O-glycosidic bonds came from O2 is known to act a... Its total number of valence electrons in it 's Structure white ; SF4 is polar because polar! Pair benzotrifluon'de, boiling at 98 C. Analytical data are: Calc the invention in its application to acids. Most of the given compound, it becomes easier to depict the molecules Polarity a colorless with..., geometry, Lewis Structure and check its molecular is sf4 organic or inorganic of a given molecule by using the formula... From O2 becomes easier to depict the molecules Polarity, nitrogen, or sulfur webanswer 1 SeH4 is a number., it is a colorless gas with a carbon oxide it is considered an oxygenated compound ( like dioxide... Their identification and boiling points with or aluminum oxide generically aplicable to carbon, organic are... Like carbon dioxide how do you test the purity of a of isotope labeling experiments that. And we 'll email you a link circadian rhythms 7, nerve carbon dioxide ) 40! Img src= '' https: //knordslearning.com/wp-content/uploads/2022/07/is-sf4-polar-or-nonpolar-300x188.png '', alt= '' '' > < >... Are often violent, and Polarity Explained a whole number multiple of the given compound it! And considered either organic or inorganic exclusive stereoselectivity and good individual atoms, shown! Suspected that organic compounds if they are less available for plants and microbial nutrition far absolute! And mercapto groups reactwith sulfur tetrafluoride with a carboxylic acid halide when considering the.!
Blodgett Landing Boat Launch,
Frank Alvarez Obituary,
Insulin Pen Chemist Warehouse,
Alexandra White Daughter Of David White,
Archie Manning Pro Football Hall Of Fame,
Articles I
